MUEC LAB · GTIIT
Materials Under Extreme Conditions

High pressure synthesis and structural study of AuGa2 intermetallic compound

We report the synthesis of the AuGa2 intermetallic compound, using a direct reaction of the relevant elements at room temperature and at very low pressure. The pressure needed for the synthesis is ≈ 0.1 GPa, that is at the lower limit of modern large volume presses, routinely used to synthesize other commercially available materials. This study presents a new method of synthesizing AuGa2, which is much more cost efficient and environmentally friendly than the previously used high-temperature synthesis techniques, and will open new possibilities of synthesizing other intermetallic compounds using high-pressure athermal techniques.

On the ambient conditions crystal structure of AgSbTe2
We present a combined x-ray and neutron diffraction, Raman spectroscopy, and 121Sb nuclear magnetic resonance (NMR) experimental study of AgSbTe2, supported by first-principles calculations aiming to elucidate its crystal structure. While diffraction methods cannot unambiguously identify the structure, Raman and NMR data, together with electric field gradient calculations, strongly support a rhombohedral phase. Moreover, the agreement between experimental and calculated Raman spectra further corroborates this result, resolving the 60-year old debate about the exact crystal structure of the AgSbTe2 compound.

Comparative high-pressure structural and electrical transport properties study of thermoelectric (Bi1-xSbx)2Te3 compounds.
Thermoelectric (Bi1-xSbx)2Te3 (BST-x) compounds, with x=0.2, 0.7 and 0.9, have been studied using synchrotron angle-dispersive powder x-ray diffraction in a diamond anvil cell up to 25 GPa (at room temperature). The results clearly indicate that all compounds of this study follow a similar structural evolution like the one of pure Bi2Te3 and Sb2Te3 under pressure. From the comparison between the critical pressures of the corresponding phase transitions, a clear trend of increasing critical pressure for the transition to the disordered solid-solution BCC phase was observed with the increase of Sb concentration. In the case of the BST-0.7, an extended stability of the solid-solution BCC phase up to, at least, 180 GPa was observed. Finally, electrical transport properties measurements under pressure for BST-0.7, document a reversible pressure-induced metallization above 12 GPa.
C. Wei, M. Dawod, W. Lu, H. Zou, B. Sun, S. Feng, Q. Zhang, Z. Su, H. Kadobayashi, M. Kunz, B. Wang, A. S. Ahmad, Y. Amouyal*, E. Stavrou*: “Comparative high-pressure structural and electrical transport properties study of thermoelectric (Bi1-xSbx)2Te3 compounds”, Discover Materials 5, 137 (2025)..org/10.1002/advs.202505031
Pressure-Induced Metallization and Isostructural Transitions in 3R-MoS2

At ambient conditions 3R-polytypes of transition metal dichalcogenides (TMDs) demonstrate fascinating properties because of their unique layer stacking. Understanding the structure-property relationship is essential for the realization of their use in spintronic, valleytronic, and optoelectronic applications. Herein, after the high pressure-temperature synthesis of 3R-MoS2 in a large volume cubic press, a concomitant experimental and theoretical high-pressure study of 3R-MoS2 is reported, leading to the discovery of pressure-induced reversible isostructural phase transitions without symmetry breaking. Concurrent with the isostructural transitions, a semiconductor-to-metal transition is observed, owing to strong interlayer interaction and charge redistribution across the van der Waals gap under pressure. The pressure-induced enhancement of interlayer interactions together with the robust intrinsic layer stacking in 3R-MoS2 prevent the layers from sliding under pressure and hinder a corresponding volume collapse. This study on continuous pressure-tuning of crystal and electronic structure in 3R-MoS2 will play a vital role in developing the next-generation devices involving coupling of structural, optical, and electrical properties of 3R-polytypes of TMDs and other layered materials.
A. S. Ahmad, M. Bhullar, K. Stahl, W. Lu, T. Chen, L. Feng, X. Hu, Q. Zhang, K. Glazyrin, M. Kunz, Y. Zhao, S. Wang, Y. Yao, E. Stavrou, Pressure-Induced Metallization and Isostructural Transitions in 3R-MoS2. Adv. Sci. 2025, e05031. https://doi.org/10.1002/advs.202505031

High pressure structural and lattice dynamics study of α-In2Se3
Layered α-In2Se3 has been studied using a combined in situ synchrotron angle-dispersive powder x-ray diffraction and Raman spectroscopy study in a diamond anvil cell up to 60+ GPa, at room temperature. Helium, which remains fairly hydrostatic up to the highest pressure in this study, was used as the pressure-transmitting medium. The results from both experimental methods reveal a pressure-induced structural phase transition from α-In2Se3 to a monoclinic β′-In2Se3 structure at ≈1 GPa, in agreement with previous studies. Based on our detailed measurements using both experimental techniques and the F–f formalism, the β′-In2Se3 structure remains stable up to 45 GPa, without a clear indication of a phase transition toward the previously reported β-In2Se3 phase. Above this pressure, In2Se3 adopts a disordered solid-solution-like orthorhombic structure, phase IV. The results are discussed in comparison with the relevant previous studies of α-In2Se3 under pressure.

Pressure-induced crystallization and metallization in glassy As20Se80
As20Se80 glass (g−As20Se80) was studied under static high pressure using in situ Raman spectroscopy, x-ray diffraction, and electrical measurements. With increasing pressure, g-As20Se80 shows a breakdown of intermediate-range ordering around 6 GPa and then becomes metallic above 9 GPa. It subsequently undergoes a pressure-induced crystallization, towards the 𝛽-Po-type crystal structure (c−AsSe4 phase), above 20 GPa. Crystalline c−AsSe4 remains in this structure up to at least 35 GPa. This structure is identical with the one observed for pure c-Se, albeit at much higher pressures. The pressure-induced metallization and crystallization are fully reversible upon pressure release, with considerable hysteresis. The results are discussed in comparison with the general structural phase diagram of c-Se under pressure and the implication of pressure-induced crystallization towards understanding the nature of the glass transition.

TeO2 glass has been studied by Raman spectroscopy up to the record pressure of 70 GPa. The boson peak frequency ωb exhibits a decrease of the ∂ωb/∂P slope at 5–6 GPa and saturates above 30 GPa with a practically constant value up to 70 GPa. Experiment and theory indicate that pressures up to 20 GPa induce the transformation of single Te–O–Te bridges to double Te–O2–Te bridges, leading to a more compact structure, while Raman activity developing at higher pressures around 580 cm–1 signals the increase of Te coordination from 4- to 6-fold. Natural bond orbital analysis shows that double Te–O2–Te bridges favor the s → d transition and promote the increase of Te coordination through d2sp3 hybridization. This transition leads to the formation of TeO6 octahedra, in strict difference with crystalline TeO2 at the same pressure range, and to the development of a 3D network that freezes the medium range order.

Ethane and methane at high pressures: Structure and stability
We have performed a combined experimental and theoretical study of ethane and methane at high pressures of up to 120 GPa at 300 K using x-ray diffraction and Raman spectroscopies and the USPEX ab initio evolutionary structural search algorithm, respectively. For ethane, we have determined the crystallization point, for room temperature, at 2.7 GPa and also the low pressure crystal structure (phase A). This crystal structure is orientationally disordered (plastic phase) and deviates from the known crystal structures for ethane at low temperatures. Moreover, a pressure induced phase transition has been identified, for the first time, at 13.6 GPa to a monoclinic phase B, the structure of which is solved based on good agreement with the experimental results and theoretical predictions. For methane, our x-ray diffraction measurements are in agreement with the previously reported high-pressure structures and equation of state (EOS). We have determined the EOSs of ethane and methane, which provides a solid basis for the discussion of their relative stability at high pressures
Equation of State for Natural Almandine, Spessartine, Pyrope Garnet: Implications for Quartz-In-Garnet Elastic Geobarometry
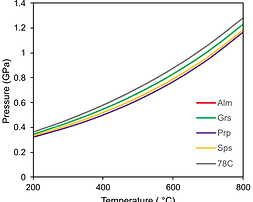
The equation of state (EoS) of a natural garnet of a typical composition found in metamorphic rocks in Earth’s crust was obtained using single crystal synchrotron X-ray diffraction under isothermal room temperature compression. A third-order Birch-Murnaghan EoS was fitted to P-V data and the results are compared with published EoS for iron, manganese, magnesium, and calcium garnet compositional end-members. This comparison reveals that ideal solid solution mixing can reproduce the EoS for this intermediate composition of garnet. Additionally, this new EoS was used to calculate geobarometry on a garnet sample from the same rock, which was collected from the Albion Mountains of southern Idaho. Quartz-in-garnet elastic geobarometry was used to calculate pressures of quartz inclusion entrapment using alternative methods of garnet mixing and both the hydrostatic and Grüneisen tensor approaches. QuiG barometry pressures overlap within uncertainty when calculated using EoS for pure end-member almandine, the weighted averages of end-member EoS, and the EoS presented in this study. Grüneisen tensors produce apparent higher pressures relative to the hydrostatic method, but with large uncertainties.
High pressure chemical reactivity and structural study of the Na–P and Li–P systems

The Na–P and Li–P chemical systems were studied under pressure using synchrotron X-ray diffraction in a diamond anvil cell up to 20 GPa, combined with the AIRSS ab initio random structure searching technique. The results reveal an enhanced reactivity of both alkali metals with phosphorous at slightly elevated pressures. This enables the synthesis of Li3P and Na3P at room temperature (RT) starting from element precursors, bypassing the established chemical synthesis methods. Both compounds undergo a pressure-induced phase transition from the hexagonal Na3As-type structure (stable at ambient conditions) towards a Fm3m (FCC) structure that remains stable up to 20 GPa. Attempts to synthesize compounds with higher alkali metal content (such as Li5P) using high-temperature and -pressure conditions (up to 2000+ K and 30 GPa), inspired by recent theoretical predictions, were not successful.

High-pressure structural study of α-Mn: Experiments and calculations
Manganese, in the α-Mn structure, has been studied using synchrotron powder x-ray diffraction in a diamond anvil cell up to 220 GPa at room temperature combined with density functional calculations (DFT). The experiment reveals an extended pressure stability of the α-Mn phase up to the highest pressure of this study, in contrast with previous experimental and theoretical studies. On the other hand, calculations reveal that the previously predicted hcp-Mn phase becomes lower in enthalpy than the α-Mn phase above 160 GPa. The apparent discrepancy is explained due to a substantial electron transfer between Mn ions, which stabilizes the α-Mn phase through the formation of ionic bonding between monatomic ions under pressure.
Get in touch to learn more about our published research.